
No part of these materials may be reproduced, distributed, or transmitted, unless for course participants.
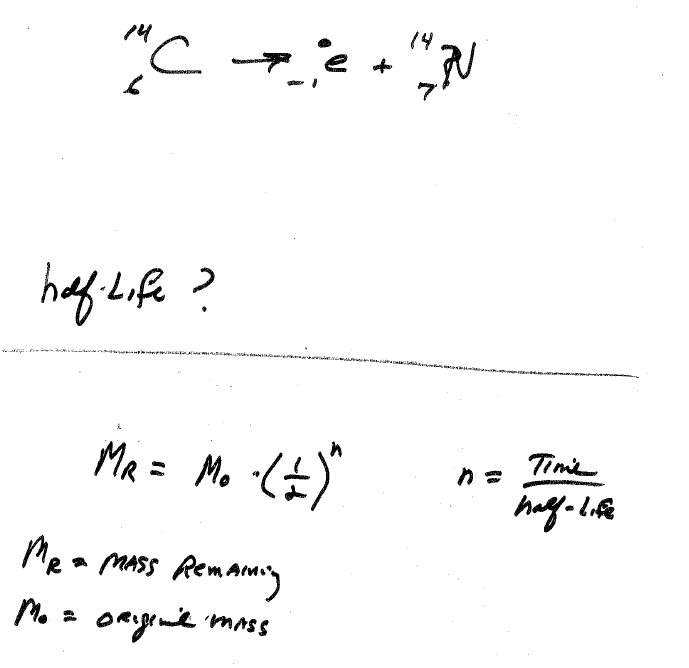
Stream the 10-hours of video recordings anytime, 24/7. Over 300 pages of course slides and additional references will be available for immediate download. Please contact Lisa Le for a certificate. control rods to control the rate at which the reaction occurs. NOTES: Concurrent enrollment in MAT 115 or. a moderator to slow down the high-energy neutrons produced in the reactions. Receive an AIAA Course Completion Certificate upon viewing all course recordings. These topics include molecular orbitals, acid-base chemistry, chemical. System design, development, testing, analysis, program management, contracts,Ĭlassroom hours / CEUs:10 classroom hours / 1.0 CEU/PDH Atmospheric mining in the outer solar systemĪudience: This course is intended for students,Įngineers, and managers involved in advanced space propulsion component and.Nuclear thermal propulsion (NTP) for Mars missions.No two radioactive isotopes decay at exactly the same rate.
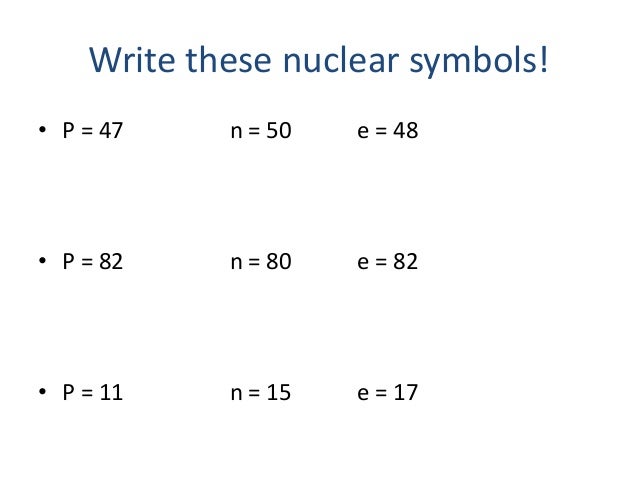
The numbers of nucleons that represent completed nuclear energy levels - 2, 8, 28, 50, 82 and 126 - are calledmagic numbers.


 0 kommentar(er)
0 kommentar(er)
